Sidebar. The odd abundance of odd-nucleon atoms
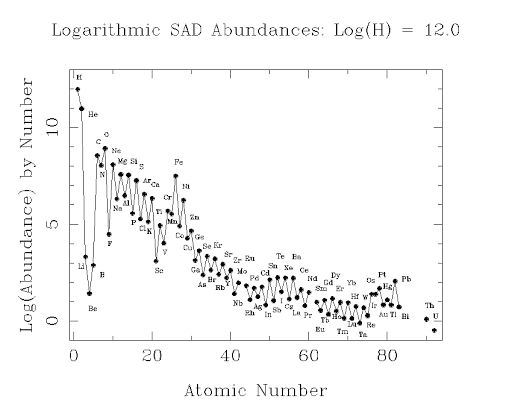
As noted in the main text, atoms of odd atomic number are notably less abundant than are atoms of even atomic number. Here’s a graph that all astronomers learn about:
UNLV Physics
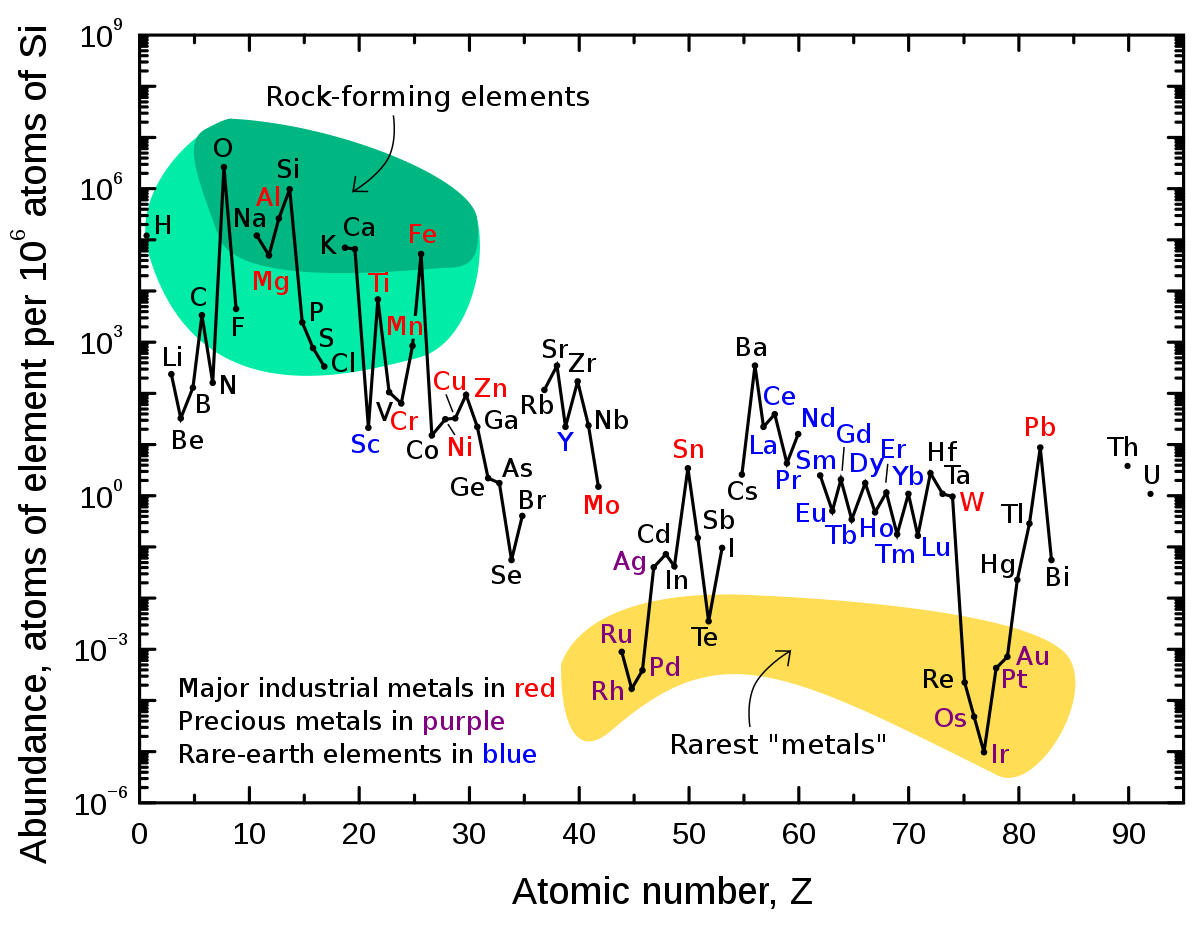
Note that this graph uses a logarithmic scale, so that a drop of one unit is a drop by a factor of 10. Thus, helium, He, is about 1/10th as abundant, and lithium, Li, is about 1 billionth (10-9) as abundant. All are keyed relative to hydrogen, the most abundant and original element in the Universe. The estimates are for the solar system. It was left with many heavier elements by a supernova or a neutron star merger before it coalesced. In the Earth’s crust the pattern is rather different, as the elements have been sorted out by many processes:
Wikipedia
Nonetheless, the pattern of lower abundance of odd-atomic-number elements remains. Jumping back into the discussion of the possible solvents for life on other planets, consider ammonia (which has its problems). Ammonia is less widely available astronomically than is water because nitrogen, atomic number 7, is made in stars in much reduced quantity than is oxygen. The next analog of water, hydrogen fluoride, is also far less abundant than water astronomically, for the same reason, F having atomic number 9.
The patterns reflect basic energy relations in the binding of protons and neutrons in atomic nuclei. Nuclei with even numbers of protons+neutrons pair nicely into energy “shells,” much as do electrons round the nuclei in neutral atoms. For the operation of the strong force, it matters less whether the partner of, say, a proton, is a neutron (n) or a proton (p). Most stable are nuclei with an even number of protons and an even number of neutrons.Carbon has 6p + 6n and is abundant, an even-even nucleus; nitrogen is 7p +7n, not too bad, but less abundant than C or O. Fluorine is 9p + 10n, and is far less abundant than its neighbors in the periodic table. Its isotope 18F is unstable, showing the strain of having lots of protons: the heavier elements all have more neutrons than protons. They have good binding from the strong force and less disruptive repulsion of the protons among themselves.
A little twist: the odd-even pattern was first reported by Giuseppe Oddo in 1914.