Sidebar. Molecules and moles
Physics often involves the discussion on individual nuclides, atoms, or molecules. In the main text we trace the energy in nuclear fusion of individual protons. Chemistry more commonly treats macroscopic quantities such as grams and moles. A mole of anything is a fixed number of them, Avogadro’s number, symbolized as N0. That’s 6.022×1023 of them, a number challenging to comprehend even if well defined. It emphasizes the smallness of individual atoms. Twelve grams of carbon-12 is Avogadro’s number of them. Then the individual carbon atom has a mass of 12g/N0 = 1.993×10-23 g.
Clearly, no one counted out that many atoms. There are various means of measuring N0. We can use physics to get the mass of individual atoms moving in a combined magnetic and electric field. The electric field imparts a velocity and the magnetic field bends it by a readily measured amount (the principle of mass spectrometry for lots of chemical identification).

Credit: National Institute of Standards and Technology
One literally shining example of the use of Avogadro’s number is in this sphere of isotopically pure silicon. The number of atoms in the sphere is known from its total volume and the volume occupied by an individual silicon atom in its crystal lattice. One then measures the mass of the whole sphere and calculates the kilogram as another fundamental unit. Multiplying the mass of the sphere by the ratio of N0/(calculated number of atoms) gives us 28 kilograms.
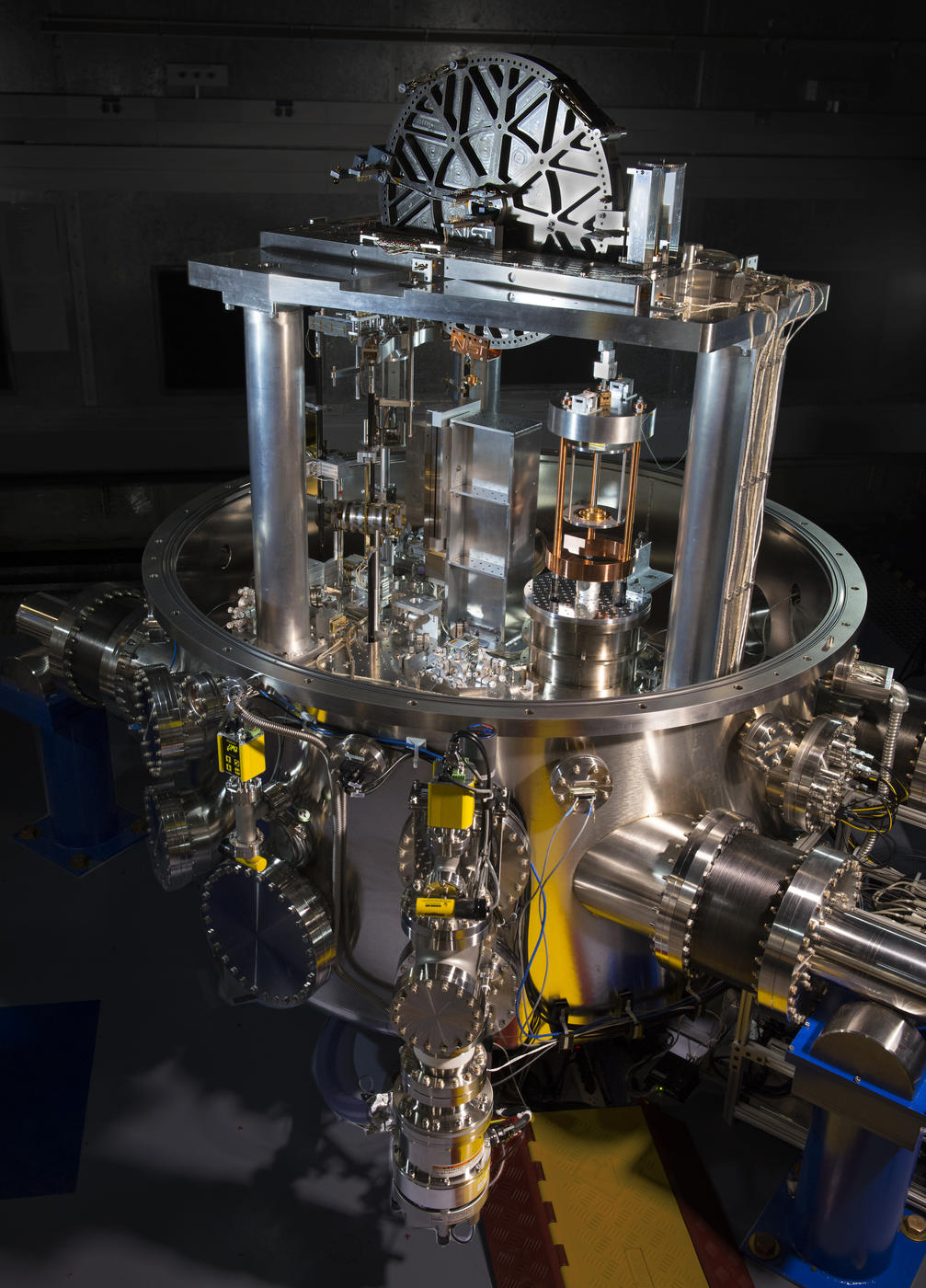
The new definition of the kilogram is not based on a single physical artifact that is kept in one place, such as the standard kilogram mass in Paris at the the International Bureau of Weights and Measures. That mass has also gained and lost tiny amounts of mass. The new kilogram is based on the Kibble balance. That’s not a device for measuring dog food but an intricate device that balances the force between two coils carrying electrical currents measurable with extreme accuracy. It can be replicated anywhere in the world with a high-tech lab and much money. Any system of metrology (measurement) must have several fundamental units chosen as fixed – that is, “rulers” in various dimensions of, say, mass, length, voltage, etc.. The variable old physical mass formerly taken as a fundamental constant now is replaced with a method that takes another item as fixed by definition, Planck’s constant. The precisions in modern standards and the intricacy of their measurement is worth a look in any of many sources, physical or electronic.
J. L. Lee, National Institute of Science and Technology
We have the concept of a mole of immaterial objects, the photons. In physiological ecology we measure the performance of plants using sunlight. Our unit of photon flux density is moles of photons per square meter per second. In our desert area that can reach 2200 micromoles (μmol) m-2 s-1. It would be quite tedious to record sextillions of photons.
The world’s oceans contain a bit more than 1/10 of an Avogadro’s number of moles of water (calculate it). It is amusing to calculate this oneself, using the mean depth of the oceans a 3682 m. However, no one uses units of moles of moles. We could name that unit the sunfish, whose zoological name is Mola mola. I have proposed also that Avogadro’s numbers of avocados is a guacamole.