Appendix. Recycling of nitrogen on Earth
I must admit that nitrogen is my favorite element in the geochemical cycle as well as in plant physiology and ecophysiology. My 1981 article, Evolved strategies of nitrogen acquisition by plants in the American Naturalist crystallized my lifelong interest in nitrogen’s role in the world. The article been cited 192 times up through 26 July 2020; it’s clear that lots of other researchers are into nitrogen. Of course, nitrogen is the most limiting plant nutrient globally, and it’s those plants (plus photosynthetic oceanic organisms) that support every other living organisms other than a few truly slow-growing and low-biomass rock-eating bacteria.. Nitrogen is the mineral nutrient used by plants in the greatest amount, comprising a bit over 2% of total dry mass in live tissue on a global average.
The Earth received the vast majority of its nitrogen from the primordial solar nebula, hence, from the couple of stars that kindly exploded in the neighborhood long ago. Tiny amounts are made currently from the beta decay of carbon-14 formed with the help of cosmic rays. Our Sun makes negligible amounts of nitrogen currently and really none of it is coming our way. Nitrogen is readily retained in the atmosphere by Earth’s gravity acting against ballistic escape. Also, Earth’s magnetic field provides a shield against the solar wind. Mars had no such luck and lost almost all of its atmosphere.
The bulk of nitrogen that’s active in biological cycles on Earth is in the atmosphere. At 78% of the molecules there it has a mass of 4×1018 kg or 5 quadrillion tonnes (7.8 tonnes per square meter, about ¼ tonne pushing down on your head alone). Mostly, N stays up there as the N2 molecule, being very poorly chemically reactive in that state by virtue of having the strongest chemical bond known. Yet it does react in several routes naturally:
- Lightning splits N2 and O2 molecules; A few N and O fragments recombine to make a ew oxides of nitrogen. This may create about 10 teragrams (Tg, million metric tonnes) each year.
- Ozone also reacts slowly with N2to make nitrogen oxides – perhaps 5 Tg annually
- Natural combustion as in wildfires may fix another 20 Tg or so.
- Nitrogen and oxygen might react slowly on desert sands.
- The very biggest natural reaction is biochemical, around 100 Tg per year. Nitrogen-fixing bacteria in a variety of species evolved an enzyme complex based on the nitrogenase enzyme, able to break the N2 bond to create ammonia ultimately. This is a slow process with a great energy cost. Here’s the rub: biologically reactive (=biologically useful) ammonia made at great cost by nitrogen-fixing organisms then becomes available to other organisms that compete with the fixers. Some Nif bacteria live alone while most are in symbioses with multicellular organisms such as the legumes or alder trees. Plant supply photosynthetically produced carbohydrates as an energy source and get a good part of the ammonia product in return. Plants also provide a controlled environment for the bacteria that has essentially no free O2 molecules. To support aerobic energy metabolism, the leghemoglobin (LHb) molecule shuttles O2 in bound, safe form. The partnership of plants and bacteria evolved so tightly that the gene to make LHb got transferred over time to the plant.
Of course, over the not-so-long term, the reactive ammonia ultimately gets turned back into N2. The major path is for ammonia to get oxidized in soil, to nitrate ion, NO3–. It migrates by hydrologic processes to oxygen-poor places in soil, water bodies and land, and anoxic ocean locations. There, other bacteria use nitrate as an oxidant /energy source, creating mostly nitrous oxide, N2O. That’s a mobile gas moving to the atmosphere to be broken down by abiolgoical processes to N2 again.
Before Fritz Haber and Carl Bosch in Germany invented the process of industrial production of ammonia, the natural processes of nitrogen fixation and denitrification balanced out at about 200 Tg per year. That’s a fraction of 50 parts per billion of nitrogen in the atmosphere. An equivalent statement is that the average N atom turns over in about 20 million years. That’s an evolutionary time scale! Nitrogen is so precious to living organisms that they have evolved great suites of traits and processes to acquire and hold onto nitrogen. I offer my paper cited above as a generally readable summary. Here is a key figure from it:

Living organisms on any habitable planet almost inescapably use nitrogen in their genetic material, protein, and other key biochemicals, notably in their photosynthetic systems. Evolution of biological nitrogen fixation appears to be a necessity for a vigorous biota. Speaking of a very (overly) vigorous biota, we humans desperately exploited nitrogen fixation. A concentrated natural resource of fixed N was sea-bird and bat guano, over which wars were fought. Two main uses of ammonia and of nitrate (readily made from ammonia industrially) are nitrogenous crop fertilizer and explosives. In WWI Germany kept going militarily in good part from the Haber-Bosch process. Globally we humans now fix about 150 Tg of nitrogen in that way. We use up 2-3% of total world energy use just for this.
While human biomass is now tops among all large animals, we embody a small fraction of reactive nitrogen in our bodies and even in our livestock and crops. Consequently, all that reactive nitrogen as ammonia and nitrate just recycles on even an annual cycle. How it recycles is quite damaging environmentally. Excess N-based fertilizer elutes for farm soils into rivers and then into the ocean at river deltas. There, along with equally problematic phosphate fertilizers, it supports great algal blooms. A big part of their problematic nature is that the blooms die off. Their decay uses up oxygen in the ocean waters. At the Mississippi River delta alone an annual die-off of virtually all marine life occurs over an area that average 50,000 square kilometers.
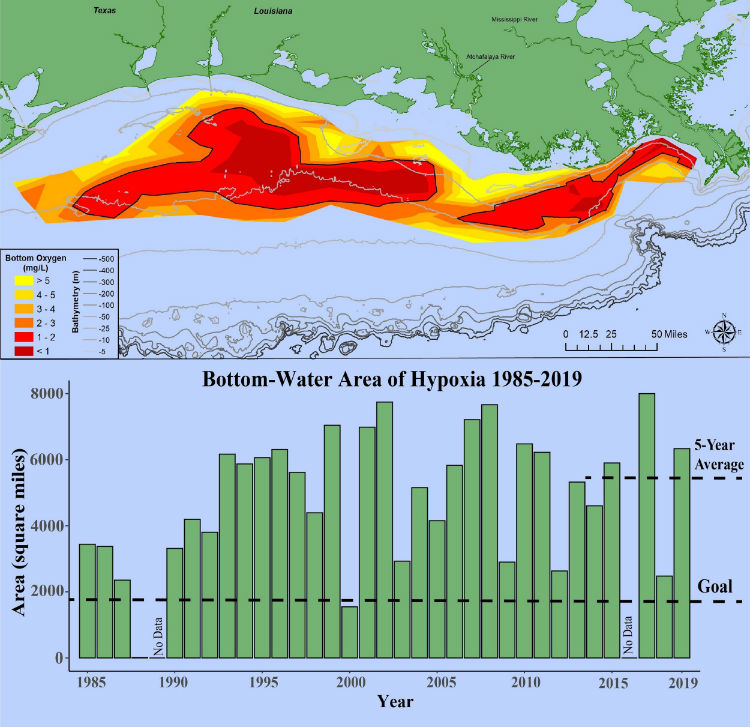
US National Atmospheric and Oceanic Administration (NOAA), August, 2019
We can’t stop ourselves from pushing the envelope. Even outside of its biological effects N fixation has its hazards. War, of course, but also industrial explosions – 30 to date. The most recent one was in Tianjin, China; 800 tonnes of ammonium nitrate exploded, causing 173 deaths and great property damage. The highest death toll was 547 in the explosion of the cargo ship Grandcamp in Texas City, Texas, from the explosion of 2300 tonnes of NH4NO3. We’re playing with fire literally and metaphorically.