Appendix. Energy balance of an organism
Or: energy, water, oxygen, metabolic, electrolyte balances
A prime measure of the state of a living organism is certainly its temperature, strongly determining its metabolic rate and posing it for survival, thermal damage, or death. For larger organisms one should say temperatures, plural, which vary from core to periphery to appendages.
An organism’s temperature is clearly related to the temperature of its environment, such as might be detected by thermal imaging of an exoplanet or our own Earth’s bits and pieces. The relationship is complex and interesting. One finds birds, the penguins, with core temperatures of 40°C surrounded by ice, snow, and air at temperatures of -50°C. Organisms, the large ones, have energetic metabolism that offsets them from their fluid environment – air or water – or the solid rock, soil, or vegetation cushion that they contact. They often have selected interception of solar radiation – e.g., lemurs sunning themselves in the morning; leaves of plants being deployed at angles for radiative gain or avoidance.

The components of thermal energy balance are readily enumerated, if sometimes dazzling in details. Rather distinct, if linked to thermal energy balance, is metabolic energy balance – the processing of high-quality energy (free energy) that keeps biochemical processes going. Later in this appendix we note other balances that organisms achieve (or else fail to leave posterity) – water, oxygen, electrolytes, osmotic potential, specific nutrients or toxics, and pH.
Let’s start with a simple case, a fully aquatic organism such as a fish, a kelp, or a plankter. Water is a medium with good thermal conductivity in free flow (considerably less so when thermally stratified, warmer on top). Organisms with low metabolic rates have their body temperature driven close to that of the water. A notable exception is the tuna with such a high metabolic rate as to be effectively warm-blooded (homeothermic). The only other major component of its thermal balance is conduction of heat from its core to the water. To retain most of the heat in blood circulation to its periphery it has countercurrent heat exchange.
The rete mirabile or wonderful net. Countercurrent flow in between arteries leaving warm body and veins returning chilled blood. The recovery of heat is substantial. By the author, 2011.
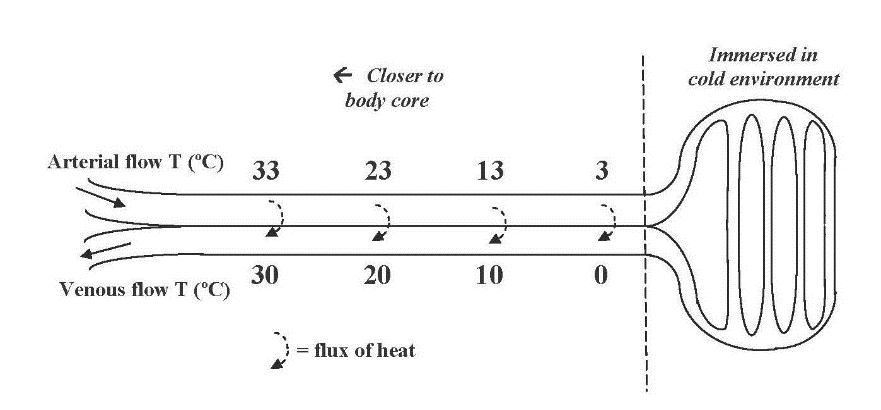
A fully analogous system lets birds wading in cold water keep their core warm.
A large, terrestrial organism has many routes of energy exchange and a source or two. Since photosynthetic organisms are the base of all food webs, we may look at a vascular plant first – that is, the plants we usually notice, with xylem vessels carrying water up (also nutrients, some hormonal signals) and phloem vessels carrying photosynthesize sugars down (also processed nutrients, some hormonal signals). Leaves, flowers, stems, and roots are in different environments with correspondingly different balances among processes of energy transport and generation. We may focus on leaves first.
By the author
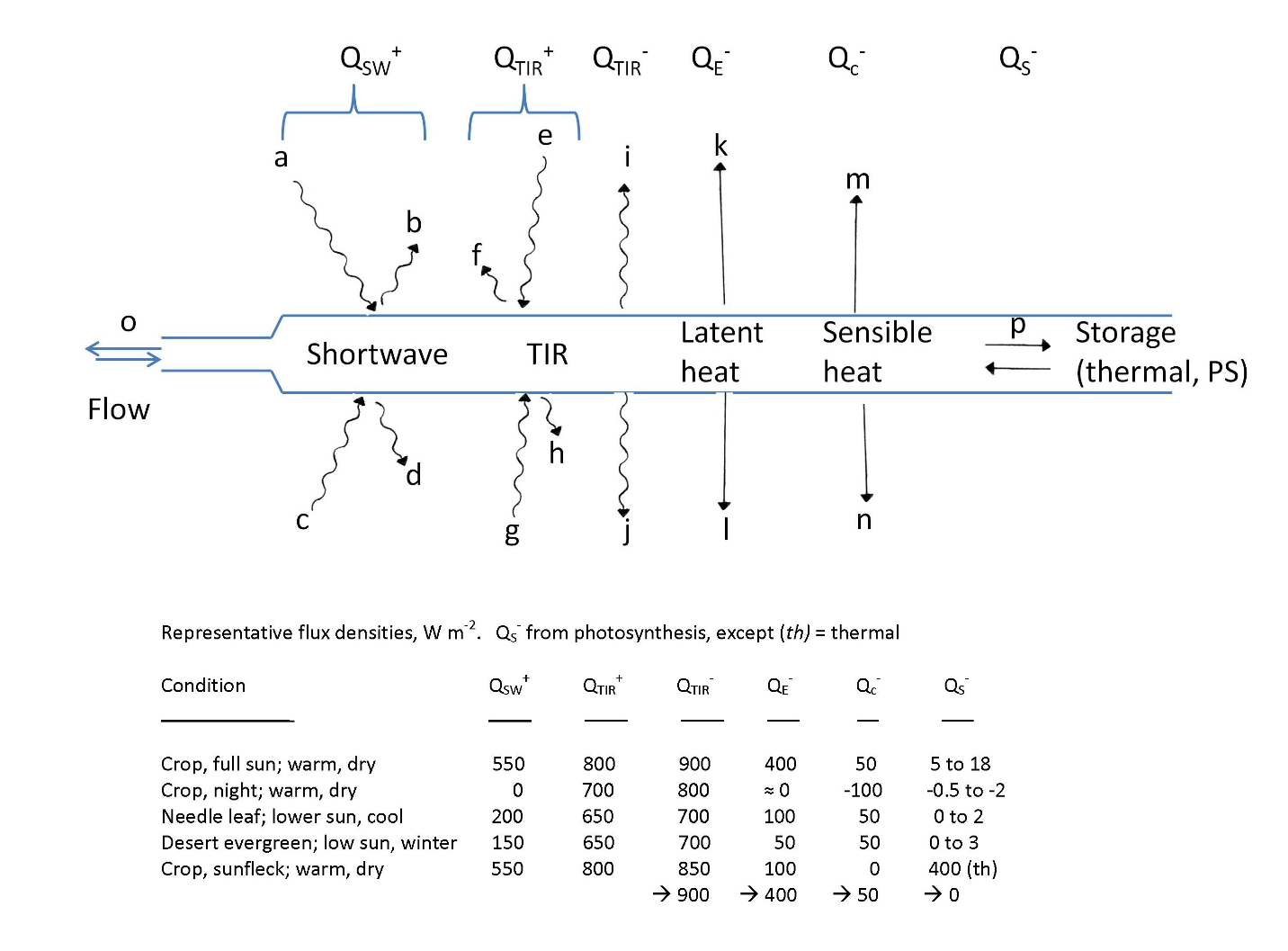
Radiation exchanges dominate the energy balance:
- Absorption of shortwave radiation in the visible and near infrared (and a little bit of ultraviolet). In full sun this can reach nearly 1000 watts per square meter – a “hair dryer” per square meter, and more than peak human metabolism. In daylight most sun-exposed leaves hit several hundred W m-2. We may resolve reflection and transmission separately.
- Absorption of thermal infrared radiation from the “material” surroundings – soil, other vegetation – and also from the sky. In what we may deem median conditions, a notional 20°C, the flux density is readily calculated from the blackbody radiation law as 418 W m-2, very comparable to shortwave flux densities. Desert soils can reach 70°C, generating a flux density 750 W m-2. A low-humidity sky can be 40°C colder as a radiant source, dipping to an effective 20°C and a flux density of 167 W m-2. Colleague Marilyn Ball led a study showing that a cold sky and cold grass leaves under a tree seedling leaves can seriously impede the function and growth of tree seedlings in what looks deceptively like a classic case of competition.
- Emission of thermal infrared radiation from leaf surfaces in both directions, also at a blackbody rate. Leaves and flowers rarely get more than about 10°C offset up or down from air temperature, so this process is fairly constrained.
Other energy transfers:
- Convective heat transfer to the air, net inward or net outward. In most conditions this is at a rate that is the product of the leaf-to-air temperature difference and the conductance of the boundary layer. That conductance rises with windspeed and falls with leaf linear dimension. In very low wind conditions free-air convection occurs with generally poor heat transfer. Very rarely will one find leaves in contact with a solid surface, transferring heat by conduction.
- Transpirational cooling: Leaves must open the pores in their surfaces, the stomata, to gain CO2 for photosynthesis. At the same time, water evaporates from wet surfaces of the interior cells. The flow of CO2 in is driven by a small difference in partial pressure of about 10 pascals. The flow out of water vapor is driven by a far greater difference in partial pressure. On a day with 50% relative humidity with both leaves and air at 30°C (a mild summer temperature in the so-called temperate zone), the partial pressure in the leaves is 4245 Pa and in the air it is half that, for a difference of 2123 Pa. That’s over 200 times the driving force for CO2, and water is also more diffusible. Plants typically exchange 200-500 water molecules for each CO2 molecule gained. The energy loss depends on the stomatal conductance. For wide-open stomata on a typical wild plant at 0.3 mol m-2 s-1 (I won’t delve into the interesting units, in the interest of brevity) and a boundary-layer conductance of twice that (decent breeze), the rate of water loss is 0.0042 moles of water per square meter per second. That carries away 187 W m-2. Some active wild plants and a number of crop plants readily reach 400 W m-2. Leaves may be notably cooler than the air. In any case, water use by plants is necessarily enormous on any scale, leaf to globe. Variously by season, plant transpiration may reach ¾ of all water flux from land surfaces.
Plants exhibit many evolved traits for conserving water. There are two “front-ends” to photosynthesis that reduce water use, the C4 and CAM pathways, which see. Plants can simply reduce stomatal conductance and water loss – and photosynthesis and growth – when water is in short supply. They may still dehydrate and overheat, suffering damage or dying. Plants on any exoplanet almost certainly operate similarly. There is no membrane that is selectively permeable to CO2 over water vapor that plants might deploy instead of open pores. Water is also the cheapest structural material to build stiff stems and leaves. Plant cells are pumped up to as much as 10 atmospheres by water pressure; they collect small molecules – ions, sugars – that generate a very low osmotic potential to draw in water at high pressure. There are many excellent expositions on plants’ water balance to explore.
There are relatively uncommon conditions that are the reverse, with water gain. The major such condition is dewfall, with a quite small energy flux. There is also evaporation from wet leaf surfaces after rainfall, offering substantial cooling. Leaf surfaces are water-repellent, limiting the amount of water accumulation for evaporation.
- Thermal storage and its converse of release: Most plants have thin leaves that have almost negligible storage capacity (they are about 1/4 mm thick; collapse all the aboveground plant biomass on a big annual crop and it will be about 6 mm depth). The only common plants with high heat storage I active tissue are succulents.
- Finally, there is metabolic energy. Plants have slow metabolisms overall.
- They store energy chemically as sugars. Over a whole growing season, this represents no more than 6% of the shortwave radiant energy falling on them (sugarcane, in optimal conditions); more typically it is on the order of a few percent, and globally only 0.3%!. There are good reasons for this – see the “appendix squared” below. At peak times for the most photosynthetically active plant, Camissonia clavoformis, the rate of CO2 capture was 40 micromoles per square meter per second. Given that a mole of CO2 makes ultimately 1/6 of a mole of glucose, that makes 6.7 μmol m-2s-1 as glucose. A mole of glucose has an energy content as enthalpy of combustion of 2.8 megajoules. Thus, this rate of energy storage in glucose is just under 19 watts per square meter. For photosynthetically active radiation (400-400 nm wavelength) hitting the leaves at over 300 W m-2, that’s again about 6% conversion, and about 2.4% for the total solar shortwave radiation.
- Plants release metabolic energy at a similarly slow rate. There are very, very few cases of plants metabolizing fast and getting warm. In fact, the only well-known case is skunk cabbage. It burns up sugars “wastefully” so fast in one limited part, the spadix, that it gets as much as 35°C hotter than the surrounding air, if the air is still. It does this for only a short time, to spread odorant molecules that attract flies that can carry its pollen or deposit pollen from other skunk cabbages.
Special parts: the roots:
- More in the way of an anecdote, I note that stems or roots of plants in desert soils have to traverse an extremely hot soil surface at times, up to 70°C. How their tissues survive and prosper has yet to be resolved.
I offer a link to one of my reviews of leaf energy balance for more details.
Tolerances and extremes: Almost all plants tolerate wide swings in temperature – between seasons between sunlit and shaded intervals, with passage of storms. Their bodies generally tolerate the changes well, unlike, say, we humans whose brains and hearts fail at depressions or elevations of 5-10°C. Not having nervous tissue that is metabolically poised in us rather exquisitely (other than in our skin and periphery), plants tolerate swings. That said, extremes of temperature do impact plant performance strongly. The accurate description of extreme events and of their consequences is a rich topic. I offer a link to a highly-cited synthesis publication that colleague Hormoz BassiriRad and I wrote in 2003.
All told, the energy balance of plants on Earth has a wealth of biophysical processes, and a wealth of constraints based on biochemistry, physics, and ecology. On any exoplanet the same biophysics applies. The biochemistry is very likely highly similar. The ecology is likely to be very different but subject to many regularities. The figurative and literal blossoming of plant life on and required unusual resource availability (light, a very active water cycle, nutrient reserves and cycling). It required the evolution of many other species, including symbiotic fungi (mycorrhizae that aid roots in gathering nutrients), symbiotic bacteria (for biological nitrogen fixation, e.g.), and, for its true flourishing, millions of species of insects as pollinators (but also herbivores, defenders from herbivores, etc.). This takes a lot of time for evolution and a surety of survival of many species of all kinds of organisms. An exoplanet with life more complex than bacteria and of vigorous activity is likely to be very rare, even with every other favorable condition of host star, orbit, prior supernovae, mass, and all the other features I call into view.
On to animals, especially large ones:
Animal bodies have the same processes acting on them as do plants, but in quite a different balance. The radiative balance is similar in form. Most of the time, however, we humans don’t try to expose ourselves lavishly to shortwave radiation – heat loading is only pleasant in cold conditions; ultraviolet exposure is risky, though necessary for our production of vitamin D.
One major difference we animals have from plants is in metabolic energy production. Small, active animals are powerhouses, the hummingbirds and the shrews perhaps most notably. While adult humans generate about 70-100W at rest and up to 600W at peak for trained athletes, that’s “only” 35 to 300 W per square meter. It’s also about 1 W per kg. The scaling with size is remarkable over the equally remarkable range of animal sizes. A report by P. J. Schaeffer and colleagues in the Royal Society Open Journal of 2020 measured metabolic rates of tiny shrews. Those with a mass of 7.5 g had an oxygen use rate of about 1 ml per minute. With the conversion to moles of O2 to 4×10-5 mol min-1, the stoichiometry of 1 mol glucose per 6 mol O2, and 2.8 MJ per glucose oxidized, we obtain 0.31 watts for the little guys. Per mass that’s 41 W per kg, about 40 times our rate! Shrews must eat constantly or die. Per body area (estimated area as 0.0037 m2 rather geometrically, the shrew’s metabolic rate is about 83 W m-2. That’s in our range as 35-300 W m-2. It has to be, for effective heat unloading. At the other end of the spectrum is the elephant, with much lower rates on both bases.
A note on metabolic power (energy per unit time), shrews and humans compared to plants: Plants seem placid but many have quite high metabolic rates per mass. Take a 1 kg crop plant. With 1/5 of its shoot mass as leaves and 5/6 of its mass as shoots (the rest as roots), it has 167 g as leaves. The leaves may have 60 g of dry mass per square meter (they are thin), giving it a leaf area of 2.67 m2. At their daytime peak of doing photosynthesis at half the rate of the aforementioned champion, Camissiona claviformis, they are storing energy at 9.5 watts per square meter (about ¼ what resting humans do) but 9.5 watts per kg, at the extreme of what humans can do. Plants are very thin overall. They use a small mass to cover a large area. A human is far more squat, with low area per mass. There are no photosynthetic animals and the only half-animal-like protists such as Euglena are all tiny. Animals of any size must rely on plants as the ultimate source of food energy. This is surely the case on any habitable exoplanet with whatever taxa are analogous to animals and plants.
This discussion on animals is all well and good for warm-blooded or homeothermic animals. Most animals, by far, are not – the insects, the reptiles, the fish. Reptiles and most fish have much lower metabolic rates then homeotherms. They can subsist on much less food per body mass per unit time than we do. The small insects are more strongly linked to convective and conductive heat transfer than are large animals. It is a real challenge, then, for a honeybee to warm up to flying temperature on a hot day.
For a wide-ranging discussion of animal energy balance, I again refer you to Campbell and Norman, An Introduction to Environmental Biophysics.
They also cover water balance, which is equally critical for individual survival and reproductive success. The big vertebrate animals on land have a water-balance challenge much as do plants. Our two biggest sources of water loss are excreta, particularly urine, and exhalation of water vapor from our lungs… that is, until we sweat. Humans can concentrate urine fairly well under stress but birds are the champions with their nearly dry droppings. Respiratory losses are modest. If we take 10 breaths a minute at rest, each breath as 0.5 liter, that’s 5 L min-1. At saturation at body temperature, our exhaled air has 6280 Pa of water vapor pressure, making 2.4 mol of water per cubic meter. or 43 mg per L. If we take in air at half that content we have a net loss of about 100+ mg per minute, 6 g an hour. With the heat of vaporization of water being about 2400 J per g at body temperature, that yields a heat loss rate of 4W. Climbers of Chomolungma breathe so heavily that they lose up to 10 time more, threatening dehydration and contributing a little to the risk of hypothermia.
Tolerance of vertebrate animals to temperature regimes is in a sometimes wild pattern. We humans tolerate cold down to freezing on our periphery. Our core – heart and many internal organs – function poorly with relatively small deviations from our “set point” that’s commonly quoted as 37°C, though likely to be about 0.5°C in a modern, healthy population. Brain and hormonal functions decline with excursions of several °C and death commonly occurs near core temperatures of 41°C on the high end or 32°C on the low end (which I reached on a rainy and cold Boy Scout hike at age 13 with a clueless scoutmaster). Several people have been revived by heroic efforts at much lower core temperatures. Skier Anna Bågenholm recovered after 80 minutes in icy water that took her core temperature to 13.7°C. Birds keep higher core temperatures around 40-41°C with perfectly normal neural function, while hummingbirds may go into energy- and life-saving torpor down to 18°C. Turtles survive near 0°C with anoxia. The variation in tolerance, and the mechanisms of physiological damage at extremes, is yet to be understood in full.
More about water balance: Sweating, of course, is a potentially copious loss route. Lou Ellen and I were at the stunning mine at Naica in Chihuahua, Mexico. The room with the gypsum crystals 1 m thick and 10 m long is at 55°C. We, as others, lost perhaps 3 L of water per hour… fortunately only for 5 min. Sweating is our human breakthrough, allowing us to run down any other animal, as noted in the main text. We tolerate water loss poorly, as do almost all organisms other than tardigrades or resurrection plants. While camels can lose 30% of their body mass and survive nicely, we humans die at 15-25% loss. The loss of electrolytes – sodium and potassium – is as critical with massive sweating loss. You may recall that water molecules are not pumped out of the sweat glands by active transport of water molecules themselves. Rather, transport proteins in the glands actively pump out ions, which then draw out water by osmosis.
The other balances noted at the beginning – oxygen, nutrients, pH, osmotic potential – all deserve whole chapters that don’t readily fit here. There are many places to discover their wonders and intricacies. They, too, are critical for habitability and certainly would take on different relative imports on exoplanets with different elemental cycles.
“Appendix squared:” why is photosynthesis so energy-inefficient?
- Plants use only part of the solar spectrum, 400 to 700 nm in wavelength. This contains only 40% of solar energy. This is a constraint of the still-remarkable photochemistry of chlorophyll.
- They absorb only about 86% of the photons in that range, variably so by species and condition. Some light is reflected, some is transmitted. In a full canopy, however, as much as 95% might be absorbed.
- Some of the light, in between blue and red, is absorbed by auxiliary pigments, which pass the energy of electronic excitation to chlorophyll with less than 100% quantum efficiency. Make it about 85% quantum efficiency across the range of 400-700 nm.
- In full sunlight, leaves are absorbing far more light than they can use photochemically in photosynthesis. Typically leaves saturate in their ability to use light energy at a fairly small fraction of full sunlight intensity, often about 1/3rd to 1/5th. This is a very interesting ecological trade-off. Plants deploy leaves for their own function but also shade competitors. They cannot build practical leaves stuffed with enough photosynthetic enzymes to use full sunlight. The sticking point is the enzyme that does the first biochemical step in taking up CO2, ribulose bisphosphate carboxylase oxygenase, or rubisco. It has the second most difficult biochemical reaction, after nitrogenase enzyme, so it is slow and must be present in large quantities in leaves.
- Some energy of electronically excited chlorophyll molecules gets diverted into loss routes – the molecules may fluoresce, they may do radiationless relaxation, they may undergo intersystem crossing to create potentially damaging triplet states. The main text goes into some detail. Give it about 90% pass-through of useful energy here.
- Only the first excited state of chlorophyll does the photochemistry. Chl molecules excited to the second excited state by blue light drop down to the first excited state, losing up to 44% of the energy.
- The energy of electronic excitation gets changed into chemical energy of reducing power. Later enzymatic steps lose energy, also. The losses are necessary for fast, irreversible pushing of energy along. From the energy of 20 incident photons (at non-saturating light levels!) only one CO2 molecule is fixed into glucose. That’s only a 33% energy efficiency.
- The steps to here get us from photons to glucose. The plant must use glucose energy for synthesizing its complex biochemical components. It must also do maintenance and repair.
With all the biophysical and evolutionary constraints it’s then very respectable that sugarcane can hit 6.6% efficiency in capturing the energy of photosynthetically active radiation (PAR, you may hear). The same low efficiency of capturing stellar energy by primary producers (“plants”) may be expected on any habitable exoplanet.